Enzymes: A Voyage of Discovery
- Scientific Articles

Thanks to a century’s worth of research, enzymes can be found everywhere, from food preparation to textile processing. And because they are such an essential fixture in modern manufacturing, it’s easy to forget that just 200 years ago we had no idea that enzymes even existed. So today we’ll be going on a little trip back in time, through the voyage of discovery that brought us enzyme technology.
While trying to refine sugar from beetroot, French chemist Anselme Payen discovered and isolated the very first enzyme cocktail (diastase) in 1833. It took another 3 years for scientist Berzilius to conceptualise the existence of catalysts, molecules that speed up reactions without getting changed in the process. However, it wasn’t until 1877 that the word enzyme was coined by Wilhelm Kühne, when trying to describe the process of yeast fermentation. For the next couple of decades scientists debated whether fermentation was a purely chemical reaction, or whether the life-cycle of yeast drove the process.
Yeast cells. Credit: Chemorse.com
In 1897, when Eduard Büchner demonstrated that dead yeast cells could perform the same fermentation as live yeast, the debate was finally settled: enzymes, inanimate chemical compounds, were capable of facilitating reactions. Around this time, chemist Emil Fischer set out to explain the mechanism of action behind enzymes, that is, how they attach so readily to their target molecules (aka substrates). He proposed the lock-and-key theory: enzymes are shaped specifically to their substrates just as a lock specifically fits its key. This explanation is still taught in school curricula to this day!
The final piece of the puzzle was solved in 1926 when James B. Sumner managed to isolate and analyse the enzyme urease, discovering that it was a type of protein; the chemical structure of enzymes was finally determined. And with that, enzymes had officially been discovered, named and explained.
Over the next few decades science didn’t slow down. The structures of the first enzymes were observed in the late 1950s, leading to further insights into how enzymes interact with substrates and aid in chemical reactions. This was closely followed by the DNA revolution. By figuring out how DNA encodes proteins and, most importantly, how this process could be manipulated, scientists were able to start designing their own enzymes.
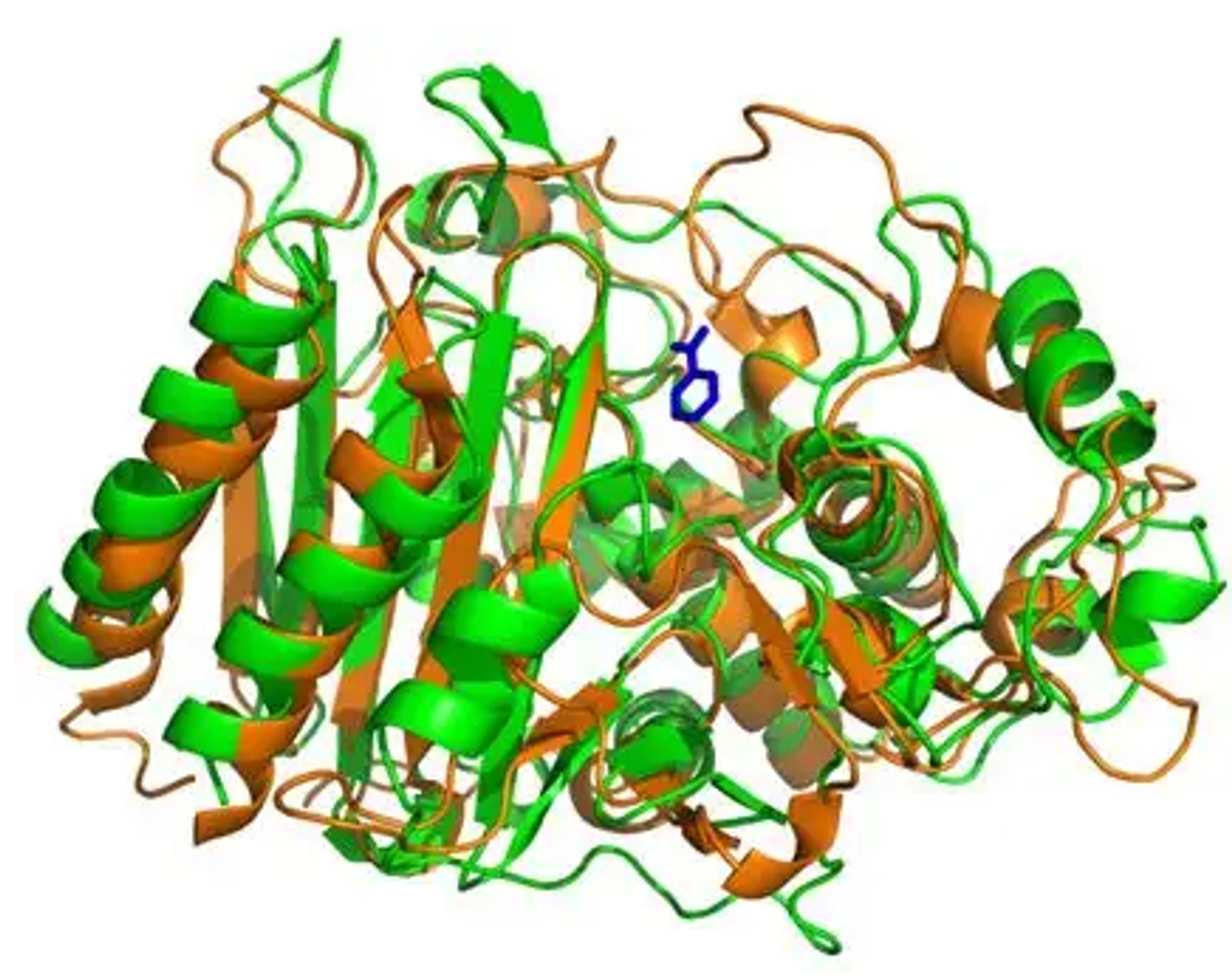
Beta-lactamase structure. Credit: HubPages.com
Changing an enzyme’s DNA means changing its structure, this affects how well an enzyme fits its target molecules and broadens the conditions in which it can operate. Suddenly we had the power to create hyper efficient enzymes, even enzymes capable of performing entirely new reactions, unobserved in nature! There are two main ways in which scientists can achieve this: creating mutant enzymes and screening them for desired characteristics, and growing organisms in difficult conditions until they create a desired enzyme variant - this is the approach Samsara Eco used to discover our plastic-eating enzymes.
In 2016, while taking samples from a landfill site, a team of scientists discovered a strain of bacteria capable of degrading one of the most common types of plastic waste: PET plastic. After closer inspection it was found that this bacteria was using a novel enzyme IsPETase to break plastic back down into its building block molecules. But while IsPETase quickly became famous for its ability to degrade one of the most persistent materials on the planet, not much was known about the evolutionary process behind it.

Sam’s scientists Vanessa Vongsouthi and Matt Spence
This is exactly what our Samsara Eco’s scientists, Venessa Vongsouthi and Matt Spence set out to investigate. By tracking IsPETase’s evolution, Samsara Eco’s scientists were able to identify important changes in the enzyme’s structure that allowed it to become the IsPETase we see today. Historic versions of the enzyme were brought back to life and screened for their stability and activity, by the end of the process two new enzyme variants had been designed, capable of surviving in harsher conditions and breaking down PET plastic more efficiently. These variants are better suited for industrial use, opening the door for wide scale, eco-friendly, infinite recycling. Learn more about how these amazing enzymes could revolutionise plastic recycling here.
And so, here we are in 2024, able to identify and engineer enzymes like never before. Samsara Eco with our super IsPETase, and thousands of other scientists working on their own enzymatic ventures. It certainly is an exciting time for enzyme technology, who knows what the next few decades have in store!